Opioid-Induced Constipation: New and Emerging Therapies—Update 2016
There has been much focus in pain management on preventing the dangerous and bothersome complications of opioid therapy, including opioid-induced constipation (OIC). One positive effect of all this attention may be the reduction in opioid-induced side effects. This review will present the latest literature on the diagnosis and management of OIC.
Opioid-Induced Bowel Dysfunction
Almost all patients requiring opioid therapy develop side effects, the most common of which affect the gastrointestinal (GI) and central nervous systems (CNS). Although tolerance develops to many of the CNS side effects over time, resolution of opioid-induced bowel dysfunction (OIBD), and more specifically OIC, does not occur with continued use.
Mu-opioid receptors, and to a lesser extent kappa- and delta-opioid receptors, are located throughout the GI tract. The large abundance of mu receptors in the GI tract contributes to the enteric nervous system, which appears to mediate the GI effects of opioid agonists by reducing bowel smooth muscle tone and contractility, which prolongs transit time.1 There is an increase in nonpropulsive contractions as well as enhanced fluid absorption. Opioid-mediated increased anal sphincter tone and decreased reflex relaxation in response to rectal distension contribute to the difficulty in rectal evacuation characteristic of OIC.
 How prevalent is OIC? The numbers vary widely based on study design and patient populations. Based on an analysis of 16 clinical trials and observational studies, OIC has been reported to occur in 15% to 90% of patients.4 When these studies are qualified according to type of chronic pain, estimates from observational studies in the United States suggested that the prevalence of OIC in patients with noncancer pain ranged between 40% and 50%.
How prevalent is OIC? The numbers vary widely based on study design and patient populations. Based on an analysis of 16 clinical trials and observational studies, OIC has been reported to occur in 15% to 90% of patients.4 When these studies are qualified according to type of chronic pain, estimates from observational studies in the United States suggested that the prevalence of OIC in patients with noncancer pain ranged between 40% and 50%.
In addition to being a common side effect, OIC significantly affects a patient’s quality of life. A study of work productivity in patients on chronic opioid therapy found that OIC impacted productivity and activity levels. Specifically, the study found that patients reported 9% work time missed, 32% impairment while working (the equivalent of 14 hours of lost productivity per week), and 38% activity impairment.
Additionally, participants in a recent OIC study commonly reported that their constipation interfered with the ability of their opioid medication to control pain, with 49% reporting moderate or complete interference, and 8% reporting that they changed how they used their opioid in order to have a bowel movement.
Diagnosis of OIC
Opioids affect the entire gut, from the mouth to the anus, and OIBD refers to the constellation of GI effects. This includes gastroparesis, gastroesophageal reflux disease (GERD), and other GI-related disorders. Although no delineation for constipation has been universally accepted, various definitions of constipation exist, and guidelines for initiating prescription therapies for OIC have been developed.
According to the American College of Gastroenterology definition, constipation is defined as unsatisfactory defecation with infrequent bowel movements, difficult stool passage, or both. Functional constipation, as outlined by the Rome IV criteria, requires 2 or more of the following symptoms to occur no less than 25% of the time over the preceding 12 weeks: straining with bowel movements, passing lumpy or hard stools; feeling of incomplete evacuation; feeling of anorectal obstruction; using manual maneuvers for facilitation of defecation; and having less than 3 bowel movements per week.
In late 2015, a consensus guidelines was published that recommended the Bowel Function Index (BFI) as a simple assessment tool with a validated threshold of clinically significant constipation. The authors wrote: “With only 3 items, the BFI15is the shortest assessment tool. Each item is scored using a numerical analog scale from 0 to 100 points. Furthermore, clinicians can quickly assess OIC severity by calculating the total BFI index score using the average score of the 3 items.” According to a study by Ueberall, a BFI range from 0 to 28.8 defined non-constipated pain patients; a = 12 point change in BFI scores would correlate with clinically meaningful changes in a pain patient’s bowel habits. For example, a reduction in BFI score from 63.4 to 28.8 (34.6 points) “would represent clear improvement in symptoms,” noted the authors.
Treatment Options
There are a variety of nonpharmacologic treatments and over-the-counter options for management of constipation, including increasing dietary fiber intake, increasing fluid intake, and increasing physical activity. Exercise has been shown to improve functional constipation; however, there is inadequate evidence to support its use in OIC, and pain patients are often limited in their tolerance for physical activity.
Perhaps overlooked in the discussions with patients is positional strategies (ie, squatting) that can help ameliorate constipation. When sitting on the toilet, the position of the puborectalis muscle clutches the rectum, thus preventing free release of waste from the anus. However, by squatting, the puborectalis muscle remains relaxed, thus straightening the pathway to the anus. This allows waste to pass easily. Squatting posture, which can be accomplished by using a stool to raise the knees while sitting on the toilet, straightens the “kink” in the colon and relaxes the puborectalis muscles.
When dietary strategies are ineffective, laxatives, stool softeners, enemas, suppositories, or even manual disimpaction are often employed. Table 1, describes categories of laxative agents.
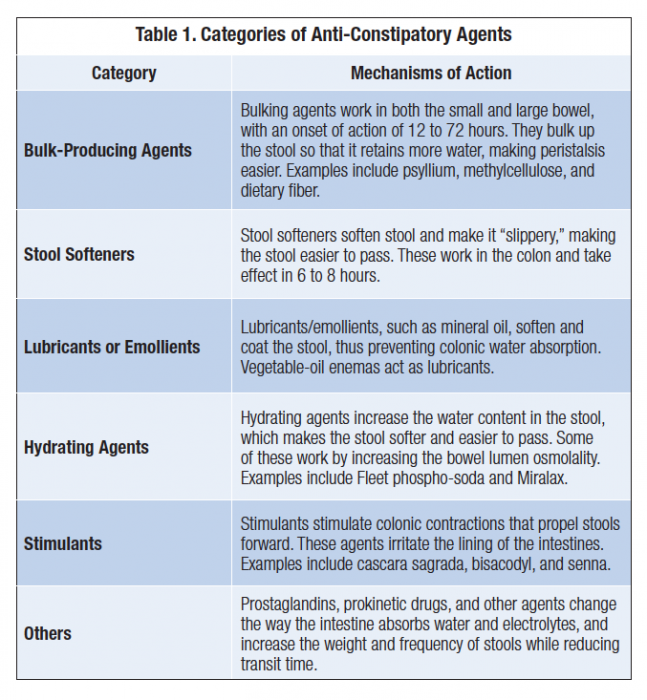
Stimulant laxatives, including senna and bisacodyl, work by increasing muscle contractions. Patients, however, may develop tolerance to and dependence on stimulant laxatives. In addition, laxatives have been shown to be frequently ineffective or suboptimal, perhaps due to their inability to directly or indirectly impact the cause of OIC at the peripheral mu receptors.



