Genetic Testing in Pain Medicine—The Future Is Coming
Over 100 million adults in the United States suffer from chronic pain on a daily basis.1 In the past decade, physicians have increasingly turned to opioids as a standard approach to pain management. Americans constitute only 4.6% of the world’s population, yet they consume 80% of the global opioid supply and 99% of the global hydrocodone supply.2 This has led, unfortunately, to a rise in opioid abuse and deaths secondary to opioid overdose. In 2014, there were 18,893 overdose deaths related to prescription pain relievers alone.
A patient’s response to pain and opioids is quite variable, making identifying patients who are good (risk-free) candidates for opioid therapy difficult. Clinicians have tried to attribute weight, age, sex, and medication blood levels to explain the wide variability of responses to opioids, but none of these factors predict a patient’s response to medications. In addition, physicians have limited tools when it comes to evaluating which patients could benefit from opioids versus nonopioids, which opioids to initiate, and/or which patients are more likely to become addicted to these opioids.

Genetic studies, however, have begun to shine light on some of these questions. This new field of medicine can potentially help physicians choose more effective treatments (similar to targeted cancer therapies), decrease iatrogenic addictions and overdoses by prescreening patients for genetic risks, and possibly even aid in the diagnosis of genetically based pain conditions.
Genetic testing can be used to explore the genes that encode the enzymes that metabolize opioid and nonopioid medications, the transporters, the receptors, and even the more cerebral aspects of perceiving and processing pain. This review covers topics at the forefront of genetics and pain medicine, including drug metabolism, addiction risk, and pain sensitivity testing.
Genetics of Drug Metabolism Leading to Personalized Medicine
Currently, the largest area of research in pain medicine and genetics has been in the field of drug metabolism. Individuals all process and metabolize drugs to differing degrees. While the practical effect of this variation can be as minimal as requiring a slightly larger dose of gabapentin, it can be quite profound when a patient has nontherapeutic levels of anticoagulation because of the way his or her body metabolizes warfarin.
Most opioid medications are metabolized by one or more of the cytochrome P450 (CYP450) isoenzymes. A patient’s ability to metabolize medications is largely based on the type and number of copies of alleles (alternative forms of genes) he or she inherits. On the one hand, the inhibition of a drug’s metabolism will result in an increase in the blood level of the parent drug and a decrease in its metabolite(s). On the other hand, induction of a drug’s metabolism results in a decreased blood level of the parent drug and an increase in its metabolite(s). People who are termed extensive metabolizers have 2 normal alleles; intermediate metabolizers have 1 normal and 1 reduced allele; and poor metabolizers have 2 mutant alleles with limited to no activity. Finally, ultrarapid metabolizers can have multiple copies of functioning alleles (Table 1).
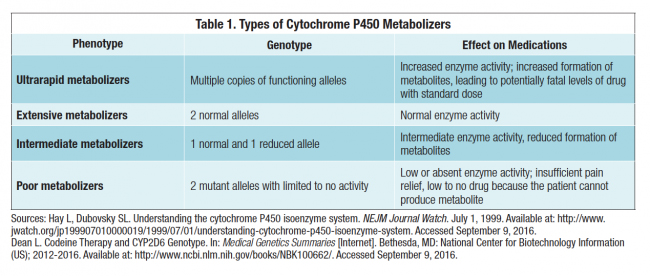
A prime example of this in pain medicine is the metabolism of codeine. Codeine is metabolized by hepatic enzymes, specifically the CYP2D6 enzyme. Codeine itself is not analgesic; it becomes effective in treating pain only when it is metabolized into morphine. Some individuals completely lack the alleles needed to produce functioning CYP2D6, and they are considered poor metabolizers. These poor metabolizers may never achieve pain relief since they cannot form the active metabolite morphine. By contrast, individuals with multiple copies of CYP2D6, ultrarapid metabolizers, can have fatally high levels of the drug on standard doses. Other opioids that are partially dependent on CYP2D6 for metabolism include hydrocodone, meperidine, methadone, oxycodone, and tramadol.
How common is this polymorphism? Approximately 7% to 10% of Caucasians are CYP2D6 deficient, while approximately 1% of Asians are poor metabolizers—explaining the slower metabolism of antidepressants and neuroleptics observed in Asians compared with Caucasians (rates for African Americans and Hispanics were not provided). Although not all opioids depend wholly on a single enzyme for activation, their effectiveness in individuals can vary substantially based on an individual’s genetic makeup. Genetic polymorphisms contribute to the variable metabolism of different enzymes. Other isoenzymes involved in metabolism of opioids include CYP3A4 and CYP2B6. Opioids that do not rely on CYP450 isoenzyme metabolism include morphine, oxymorphone, and tapentadol.
Not only does variability of alleles account for changes in drug metabolism, differences of genetic transcription of enzymes involved in drug metabolism can have an effect on the expression of enzymes. The transcription of a number of different proteins, drug receptors, and transporters can help us accurately tailor medications on a patient-to-patient basis.
One example is the m-opioid receptor gene (OPRM1) that is responsible for encoding the m-opioid receptor. Liu and Wang examined the A118G polymorphism in OPRM1 in cancer patients who developed painful neuropathy following oxaliplatin chemotherapy and were treated with tramadol (Ultracet). They reported that different genotypes (AA, AG, and GG) were directly correlated with opioid requirements for pain relief 24 hours after surgery. Patients who had at least 1 G allele (genotypes AG or GG) required much higher doses of tramadol to achieve pain relief postoperatively—pretreatment and posttreatment visual analog scale (VAS) scores were 3.1 and 2.6, respectively—requiring rescue analgesia. However, patients with the AA genotype had a better analgesic effect—pretreatment and posttreatment VAS scores were 3.0 and 0.9, respectively.
Opioid Addiction
Patient who use opioids may be at risk for addiction. Addiction involves loss of control, continuation despite significant negative consequences, and preoccupation with obtaining, using, and recovering from the effects of the drug.Opioids are used for acute and chronic painful conditions, from postoperative surgical pain to cancer pain to musculoskeletal pain. While there is a place for opioids in pain control, patients must be chosen with care. Certain patients will be prone to addiction when exposed to opioids. In such cases, the benefit of pain relief from opioids may be outweighed by the potential for addiction.
Studies of family members and twins have shown that genetic factors contribute to addictive behavior. Tsuang et al performed analyses on twin data that included 3,372 male twin pairs. In these studies, 43% of the variance in opioid abuse was attributed to genetic factors unique to that category of drug and not shared with other drug categories. Also contributing to the vulnerability to addiction were family environment and nonfamily environmental factors. Interestingly, heroin had larger genetic influences unique to it than did any other drug, while marijuana was more influenced by family environmental factors.
After establishing that genetic factors contribute to risk of addiction, investigators began to search for specific genetic differences that contributed to addiction potential. Dopamine is a neurotransmitter in the human body that is responsible for a number of tasks, including movement and pleasure. Dopamine’s effect on the mesolimbic dopamine system has been shown to be associated with traits of addictive behavior. Hence, the genes encoding dopamine and its corresponding receptors have been key candidates in the search for specific polymorphisms involved in opioid addiction.
Polymorphisms of the D2 dopamine receptor have been shown to be associated with vulnerability to addiction. Hou et al investigated the potential association of the dopamine receptor D2 gene (DRD2) TaqI RFLP A (rs1800497) polymorphism with heroin addiction. In a study including 530 heroin-addicted individuals and 300 controls, the researchers concluded that patients with an A1 allele (genotypes A1A1 and A1A2) of the rs1800497 polymorphism consumed twice as much heroin as patients without the A1 allele. In other research on the rs1800497 polymorphism, Lawford et al studied 95 Caucasian opioid-dependent patients and found that the A1 allele was present in 19% of this population, compared to 4.6% of patients without any history of abuse or addiction.
In addition, studies evaluating the D3 and D4 dopamine receptors have been conducted to look at traits that we believe make a patient more prone to addiction. Duaux et al studied a polymorphism in the dopamine receptor D3 (DRD3) gene, which encodes the D3 dopamine receptor that is in the nucleus accumbens—an area of the brain involved in reward and reinforcement behavior. Their study of 54 opioid-dependent patients and 70 control subjects concluded that patients with high sensation-seeking scores were more likely to be homozygous for a specific DRD3 allele than patients with lower scores.
Additionally, studies investigating the genes transcribing opioid receptors have been of interest to researchers. Although polymorphisms of the OPRM1 gene have been shown to be directly correlated with opioid requirements, Bond et al could not find significant differences in allele frequency between opioid-dependent and non-dependent subjects. However, in a subgroup analysis, the researchers reported that Hispanic subjects with opioid dependence had a significant high allele frequency of the variant allele of the A118G polymorphism. A similar study performed by Bart et al also showed a high frequency of the A118G variant allele in a population of 139 Swedish subjects.
As our scope of understanding grows, we will hopefully be able to use genetic testing as a tool to identify patients at risk of opioid addiction prior to starting them on opioid medications.
Pain Sensitivity
The focus of genetics in pain medicine pertains not only to medications but also to predicting patient response to pain. It is clear that not all individuals develop chronic pain from equal insults. For example, not all patients with shingles develop postherpetic neuralgia, and not all patients with diabetes develop painful peripheral neuropathy. Although it is difficult to control for cultural and environmental factors that may influence a patient’s response to pain, there seems to be a genetic component to an individual’s response to pain or the likelihood of developing chronic pain.
One enzyme at the forefront of pain perception, and the possible subsequent development into chronic pain, is catechol-O-methyltransferase (COMT). COMT is an enzyme involved in degrading catecholamines such as dopamine, epinephrine, and norepinephrine. These catecholamines, when elevated throughout the body, can cause a heightened response to pain. Individuals with lowered enzymatic activity have been shown in human and animal models to have increased pain sensitivity. A study of patients with fibromyalgia found that these patients tended to have lower levels of COMT activity. Individuals with low COMT activity, and hence higher catecholamine levels, and high pain catastrophizing preoperatively, are often more sensitive to pain and require more morphine after surgery.
A study by Rut et al analyzed the relationship between genetic variations in COMT (single nucleotide polymorphisms [SNPs] rs4680:A > G [Val158Met], rs6269:A > G, rs4633:C > T, rs4818:C > G) and pain sensitivity in patients who were undergoing lumbar spinal surgery for single level disc disease. This study followed 176 cases preoperatively and 1 year postoperatively. An association was observed in patients with the rs4633 CC and rs4680 GG genotypes, who demonstrated greater reduction of low back pain 1 year after surgery.
Another study, involving 149 children undergoing adenotonsillectomy, analyzed the same 4 SNPs within COMT analyzed by Rut et al: rs6269, rs4633, rs4818, and rs4680. The authors found that child-to-child variation in postoperative pain perception and need for pain medications was associated with all 4 COMT SNPs. Patients with minor alleles, and hence decreased COMT activity, were approximately 3 times more likely to require analgesic interventions compared to patients homozygous for the major alleles.
Although these studies are promising, it is not yet possible to confidently make clinical decisions based solely on COMT variability. However, COMT genotyping may serve as a tool to indicate which patients may benefit from a procedure or, hopefully in the future, medications to help increase the activity of COMT.
Personality Traits
Inherited personality factors have also been investigated as potentially increasing a patient’s sensitivity to pain. Life stressors and the inability to adequately cope with them can exacerbate pain for many patients. They subconsciously start catastrophizing by focusing and magnifying painful sensations. Catastrophizing is a cognitive process characterized by a lack of confidence and control and an expectation of negative outcomes. Pain catastrophizing is a negative cascade of cognitive and emotional responses to actual or anticipated pain—magnification, rumination, and feelings of hopelessness.
A recent meta-analysis concluded that higher pain catastrophizing often is associated with higher self-reported pain and disability and may lead to delayed recovery in patients with low back pain. A separate meta-analysis
determined that decreased catastro-phizing during treatment for low back pain was associated with better outcomes.
Now researchers are beginning to look at potential genetic roots for pain catastrophizing. Kuhnen et al have associated the presence of the short allele in the promoter region of the serotonin transporter gene (5-HTTLPR) with certain behaviors.25Patients with this short allele had an increased likelihood of having high levels of anxiety, vulnerability, and negative emotions. These patients had an increased chance of pain catastrophizing.
Depression, anxiety, poor sleep, and pain catastrophizing are all traits that worsen pain perception and clinical outcomes. Screening for these traits will help identify patients who may be at higher risk for developing chronic pain and poorer quality of life outcomes.
Personalized Medicine
Genetic testing is also used to diagnose painful conditions. A study by Geha et al was able to personalize treatment for the chronic pain condition erythromelalgia.26 The etiology of erythromelalgia is in the genetic mutations affecting the dorsal root ganglion (DRG) that cause one’s pain sensing to go into overdrive, causing extreme burning pain with minimal stimulation. By analyzing 2 patients’ genomic mutations, they predicted the efficacy of carbamazepine on firing of DRG neurons carrying mutant sodium channels (NaV 1.7). Carbamazepine would be effective only in patients with a specific S241T NaV 1.7 mutation, hypothesized the researchers. A double-blind, placebo-controlled study was performed consisting of 2 patients with the same mutation. Both of these patients responded favorably with a significant decrease in mean time in pain with carbamazepine therapy.
This study was limited to just 2 patients; however, as the genetic database grows, we will see a rapid growth in the number of patients in which genetic testing can facilitate both diagnosis and tailored treatment.
Conclusions
The benefits gained from personalizing pain management are just beginning to be realized. With genetic testing, patients can be placed on more effective treatments in a shorter amount of time. The trial and error process that so often is the start of treatment can be expedited, and efficacious drugs can be ordered immediately. Early and effective treatment of pain can lead to less time off work, improved quality of life, and potentially decreased chance of developing chronic painful conditions. However, genetic testing for pain is still in its infancy, and physicians must be cautious when interpreting the results. Inaccuracies will exist, and it will be possible to make assumptions and judgments of a patient’s risk of opioid addiction and pain sensitivity that would not be warranted and would raise ethical issues. Yet, in a field where cures can be sparse and therapies can be hit or miss, genetics in pain medicine could have a profound impact in the way we practice medicine.



